Cardiac manifestations and sequelae of gastrointestinal disorders
July 2009 Volume 16, Issue 4 Br J Cardiol 2009;16:175–80
Authors:
Charlotte Manisty, Ynyr Hughes-Roberts, Sam Kaddoura
The relationship between cardiac and gastrointestinal disease is widely regarded as being a complex one – disorders of the two systems commonly co-exist, and the symptoms of angina pectoris are notoriously difficult to differentiate from gastro-oesophageal problems. In addition, it has commonly been observed that patients suffering with gastrointestinal disturbance suffer from cardiac symptoms whose aetiology can be attributed to their primary gut problems. Recent evidence has shown that this is a relatively common manifestation and that the incidence of these secondary cardiac complications has been underestimated in patients with gastroenterological disease. This article reviews the evidence for secondary cardiological complications of gastrointestinal disorders and discusses the potential mechanisms behind them. The three main areas outlined in the review include rhythm disturbances related to oesophageal disease, linked angina and the cardiac manifestations of inflammatory large bowel disorders.
Rhythm disturbance and syncope related to oesophageal disease
Disturbances in cardiac rhythm accompanying swallowing are well documented, and while bradyarrhythmias including atrioventricular block,1,2 sinus bradycardia and ventricular asystole3are more common, swallowing-induced tachyarrhythmias have also been described, such as atrial fibrillation.4,5 Deglutition syncope is characterised by loss of consciousness on swallowing; it has been associated not only with ingestion of solid food, but also with carbonated and ice-cold beverages, and even belching. Evaluation of these patients has revealed that many of them suffer from underlying gastrointestinal disorders including gastro-oesophageal reflux disease, hiatus hernia, achalasia and gastric diverticulae.6Ectopic beats and tachyarrhythmias can be triggered by many of the above stimuli, but generally occur in patients with no oesophageal abnormalities.7 Both types of arrhythmia can be reproduced experimentally in these patients by swallowing a bolus of food, or following inflation of a rubber balloon situated in the lower oesophagus.
Linked angina
Oesophageal pain commonly mimics and co-exists with angina pectoris of cardiac origin: in one study over 50% of patients with angiographic evidence of coronary artery disease were subsequently shown to have gastro-oesophageal dysfunction – a higher incidence than in the general population.8 In addition, many patients with proven oesophageal disease have electrocardiographic (ECG) abnormalities, and it has been shown that typical angina pain can be elicited by instillation of acid into a patient’s oesophagus.9 This led Smith and Papp to describe ‘linked angina’ whereby gastrointestinal factors can induce angina – like chest pain and ECG changes in patients with proven coronary artery disease.10-12 It had previously been shown that raising the pressure in the bile ducts of patients with angina could provoke typical angina pain, and that hiatus hernia correction could lead to symptomatic relief of co-existent symptoms of ischaemic heart disease.11 More recently, it has been demonstrated that administering acid into the oesophagus of patients with coronary artery stenosis will reduce their exercise tolerance levels.12 It has therefore been postulated that there exists a neural reflex capable of altering cardiac blood flow, or cardiac pain perception on stimulation of the oesophageal mucosa. Manfrini et al.13 describe ischaemic ECG changes at times of oesophageal spasm in variant angina patients. They postulate that oesophageal spasm and coronary spasm are mutual and reciprocal.
Intestinal disease and cardiac complications
In addition to the association of gastro-oesophageal disease with cardiac complications, disorders of the large bowel can also result in secondary cardiac arrhythmias and pericarditis. The most commonly observed examples of this are in patients suffering from inflammatory bowel disease – more commonly ulcerative colitis14 than Crohn’s disease.15 There have been several case reports of these patients presenting with collapse due to arrhythmias – complete heart block16 and Wenckebach atrioventricular block14 have both been described. In the majority, symptoms and signs of pericarditis have also been present, and on colonoscopy there has been evidence of active inflammatory change in the bowel. It is now becoming accepted that pericarditis, with or without arrhythmia, may be a more common complication of inflammatory bowel disease than first anticipated.
Mechanisms
Rhythm disturbance with oesophageal disease
Deglutition syncope is recognised as being a neurally mediated syncopal syndrome with inappropriate bradycardia resulting in loss of consciousness on swallowing. Distension of the oesophagus with a balloon can provoke the arrhythmia and it can be abolished by atropine.1Swallow syncope has been successfully treated with both anticholinergic drugs and partial oesophageal denervation, as well as with demand pacing. Consequently, the mechanism behind swallow syncope was thought to be the result of hypersensitive mechanical receptors in the oesophagus triggering an autonomic vaso-vagal reflex resulting in a parasympathetically-mediated negative chronotropic effect. It is, however, uncertain whether physical stretching of the oesophageal walls is necessary to provoke the abnormal reflex, as carbonated or ice-cold beverages can also cause bradycardias.17 In many of the experiments, it was also not essential to inflate the balloon sufficiently to distend the oesophageal walls, in order to cause syncope,3suggesting that mechanical stimulation of the oesophagus may not be the afferent trigger of this phenomenon.
Recently, an unusual case of swallow-induced syncope was described.18 A patient receiving enalapril for hypertension suffered sinus bradycardia followed by loss of consciousness, shortly after deglutition. Continuous haemodynamic monitoring was performed for the first time in a case of swallow syncope, and revealed a transient increase in blood pressure followed by profound hypotension, and finally bradycardia. Consequently, the authors proposed that the mechanism behind this was sympathetic stimulation followed by cholinergic vasodilatation or release of humoral vasodilators (agents such as nitrous oxide and adrenaline). They also postulated that as bradycardia occurred later than hypotension, it was simply a secondary phenomenon, as a result of decreased cardiac filling stimulating the Bezold-Jarisch reflex. As angiotensin-converting enzyme (ACE) inhibitors have been shown both to facilitate parasympathetic activity and increase bradykinin (itself a vasodilator), the patient’s enalapril was withdrawn, with immediate symptomatic relief. This alternative mechanism proposed for deglutition-syncope would not explain how heart block or ventricular asystole occur secondary to swallowing, but does highlight the necessity of regular review of medication in patients suffering from swallow syncope.
The mechanism behind tachyarrhythmias triggered by swallowing is less clear, with much debate surrounding its electrophysiological basis. It has been demonstrated in some cases that the tachyarrhythmia can be triggered by paced atrial ectopic beats suggesting that atrial ectopics can trigger atrioventricular (AV) re-entry tachycardias.19 However, in most cases, because the tachycardia continues even in the presence of AV block,20 enhanced automaticity is thought to be the mechanism behind the arrhythmia.
Equally difficult to elucidate is the trigger for the afferent loop of the reflex leading to the tachycardia. One proposed mechanism is that of direct mechanical stimulation of the left atrium by the distended oesophagus on swallowing a bolus of food. This is supported by the observation that in these patients, inflation of a balloon in the oesophagus at the level of the left atrium leads to tachyarrhythmias that disappear on deflation.4 However, this concept is contradicted by evidence suggesting that balloon inflation away from the level of the heart can also trigger abnormal rhythms.21 In addition, dry swallowing can reproduce the arrhythmia, and it can be abolished by the application of local anaesthetic spray to the pharynx.21 There have also been cases reported of painful stimuli, hot and cold foodstuffs and oesophageal acid installation provoking arrhythmias.
A more commonly accepted view is that the initiating mechanism is a neural reflex resulting from autonomic stimulation. It has been shown that in some patients, increases in vagal tone can paradoxically cause tachycardias. It is therefore plausible that a parasympathetically-mediated mechanism is involved in all deglutition arrhythmias; causing bradycardias in some, and tachycardias in others.22 Despite this, it appears that in some patients the parasympathetic nervous system is involved, whereas in others, activation of the sympathetic nervous system is causative. One particular study describes three distinct autonomic reflexes resulting in deglutition tachyarrhythmias;21 while the arrhythmia was abolished by atropine and potentiated by cholinergic agonists and propranolol in one patient studied, the reverse was found to be true in another. In another patient, administration of both atropine and propranolol was necessary to abolish the arrhythmia produced on swallowing – suggesting roles for both the parasympathetic and sympathetic nervous systems.
What is clear from all of the studies on deglutition arrhythmias is that the exact pathology and aetiology have yet to be determined, although autonomic reflexes are almost certainly involved. There is no established therapy for deglutition tachyarrhythmias; therapies administered with some degree of success include verapamil, amiodarone, quinidine and beta blockers, however, a treatment that can abolish the cardiac abnormalities in one patient may potentiate them in another. Surgical repositioning of the oesophagus has terminated these arrhythmias experimentally, but this could be due either to relief from the mechanical stimulation of the left atrium by the oesophagus, or from surgical cardiac denervation.23Anecdotally, many clinicians have reported a marked reduction in rhythm disturbances with the introduction of proton-pump inhibitors in these patients – this is a therapeutic option that requires formal investigation (figure 1).
Linked angina
The differentiation of angina pectoris from the pain of gastro-oesophageal aetiology is a common diagnostic dilemma, particularly as oesophageal pain can, like that from angina, be provoked by exercise or emotion, radiate down the left arm and be relieved by nitrates. Indeed, evidence shows that exertion can stimulate demonstrable gastro-oesophageal reflux that is absent at rest,24 providing a potential mechanism for patients suffering from the pain of angina pectoris with normal coronary angiograms. The difficulty in distinguishing whether pain is of cardiac or gastro-oesophageal origin is thought to be due to abnormal visceral pain perception.25 Many visceral pain receptors are polymodal, and will respond not only to acid or mechanical distension, but also to thermal activation. Additionally, there is an overlap in the distribution of cardiac and oesophageal afferent sensory innervation entering the spinal cord.26Consequently, stimulation of the oesophagus or heart may be perceived over the dermatomes of myotomes corresponding to either organ. There may also be summation of visceral pain input from different organs leading to exacerbation of angina pectoris of cardiac origin by the symptoms of gastro-oesophageal disease.
The relationship between gastrointestinal and cardiac disease has further been complicated by ‘linked angina’ – the concept whereby gastrointestinal disorders lead to chest pain and ECG changes in patients with angiographically-proven coronary artery disease. Much evidence has validated the existence of linked angina, with oesophageal acid instillation being shown to reduce exercise tolerance in patients with angina.12 Bile duct distension has also been shown to produce symptoms of angina pectoris, while these have been completely alleviated by hiatus hernia repair. However, no one has developed a definite plausible mechanism behind linked angina, and so it is still a somewhat hypothetical proposal. It has been suggested that the sympathetic response to oesophageal pain increases myocardial oxygen demand sufficiently to provoke the symptoms of angina.27 However, more conclusive evidence in dogs has demonstrated a parasympathetic basis to linked angina, where distension of the stomach led to decreased coronary blood flow – a result that did not occur after vagal section or atropine administration.28 This suggests that gastrointestinal irritation could activate a vagal reflex leading to coronary vasoconstriction.
Evidence for linked angina in humans was produced by Chauhan et al.,29 who demonstrated that the administration of acid to the distal oesophagus in patients with angiographically-proven coronary artery disease would often produce chest pain typical of angina pectoris. They showed that the mechanism behind this pain was a significant reduction in coronary blood flow, which did not occur on infusion of saline into the oesophagus. These vascular alterations were also not seen in a group of patients who had received heart transplants, and therefore had no cardiac autonomic innervation.30 The decreased blood flow in patients with coronary artery disease was not related to any change in the epicardial coronary artery diameter, which suggested an increase in the microvascular resistance as the causative mechanism. However, there were no significant differences in the catecholamine and endothelin-l levels in blood samples taken from the coronary sinuses on acid instillation, in either group. Systemic release of vasoconstrictor substances as a potential cause of the rise in microvascular resistance was ruled out, because of the lack of generalised haemodynamic change noted throughout the experiments, and the stability in the levels of vasoconstricting agents measured in the coronary sinuses. However, the cardio-oesophageal reflex could increase the microvascular resistance directly by vasomotor nerve impulses transmitted in the vagus nerve, or by the local release of vasoconstrictor molecules.
In patients with atherosclerotic coronary vessels, vasodilating substances including acetylcholine have been shown to produce paradoxical vasoconstriction,31 and consequently it is possible that vagal activation could result in an increase in microvascular resistance. Therefore, in patients with proven ischaemic heart disease, linked angina could result from a vagally-mediated reflex causing abnormal coronary vasoconstriction, and decreased coronary blood flow.
One criticism of this study is that none of these patients had received oesophageal function studies, meaning that, potentially, their ‘angina’ pain could be of oesophageal origin, and that the decreased coronary blood flow was purely incidental. This is supported by the absence of any ECG changes in these patients even during periods of chest pain and decreased blood flow, despite previous positive exercise tests. In order to establish a causal link between gastro-oesophageal reflux and chest pain of cardiac aetiology through linked angina, studies need to use simultaneous 24-hour ambulatory ECG monitoring and pH recording. This would demonstrate that there is a temporal relationship between the acid reflux and the onset of chest pain and ECG changes. It has been argued in the past that oesophageal acid instillation is non-physiological and may have effects distinct from those in genuine oesophageal reflux disease, and therefore using ambulatory monitoring would remove this criticism. Hick et al.32 investigated patients with chest pain but angiographically normal coronary arteries using 24-hour monitoring of ECG and pH, and found no changes in parameters of reflux or oesophageal motility associated with symptoms of angina. Ischaemic ECG changes also did not correlate with episodes of chest pain or gastro-oesophageal reflux. Lam et al.33 studied patients with evidence of coronary artery stenosis on angiograms who had been admitted into a coronary care unit with suspected unstable angina. They then used 24-hour ECG and pH recordings to show that in only one patient was chest pain preceded by a drop in pH of the oesophagus – a prerequisite to linked angina. This has led authors, including Valori,34 to be sceptical about the existence of linked angina and the relevance of gastro-oesophageal reflux disease with ischaemic heart disease and angina pectoris.
Bowel disease and cardiac complications
Several cases have been described of patients suffering cardiac complications of inflammatory bowel disease, including arrhythmias and pericarditis. However, as yet, the mechanism behind these secondary cardiac events is entirely unknown. Although it is possible that the attacks of inflammatory bowel disease and the cardiac changes were purely coincidental, it is difficult to explain how systemic corticosteroid treatment resulted in a rapid resolution of the cardiac symptoms. Viral causes of pericarditis were also deemed unlikely, because levels of circulating immune complexes and viral antibodies were measured and found to be normal – both during the symptomatic phase of the illness, and during convalescence.15 In addition, ischaemic heart disease was ruled out as a potential cause of the pericarditis as both resting and exercise ECGs were without characteristic changes.
It was postulated by Ballinger et al.14 that the secondary pericarditis with Crohn’s disease and ulcerative colitis was due to an arteritis of the nodal vessels resulting, not only in the inflammatory changes seen in the heart, but also in the arrhythmias. Cutaneous (and more rarely systemic) arteritis is associated with inflammatory bowel disease, but there is no further evidence to support this claim.
One proposal for the mechanism behind the symptoms of irritable bowel syndrome (IBS) is that put forward by Smart and Atkinson,35 of abnormal vagal function. They assessed alimentary and cardiac autonomic nervous system function in patients with IBS, and showed that the response of the lower oesophageal sphincter to abdominal compression was decreased in those with IBS, and that gastric secretion secondary to hypoglycaemia was impaired in a proportion of the patients. In addition, they demonstrated an abnormality of pulse rate variability with respiration in more than 25% of patients with IBS, indicating that it is not a localised disease, but that the bowel symptoms are part of a more widespread autoimmune disorder. It would, therefore, be interesting to investigate cardiac function in patients with IBS and to assess whether there are any abnormalities that occur disproportionally – commonly in this group as opposed to the general population.
Conclusion
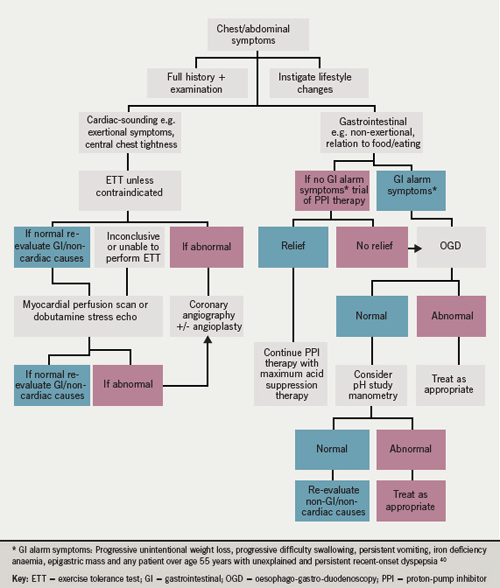
Cardiac and gastrointestinal diseases interact in a complex way. Well-known associations exist, such as that between aortic stenosis and gastrointestinal angiodysplasia (Heyde’s syndrome), the mechanism of which is thought to relate to acquired deficiency of von Willebrand factor.36-38This review article has aimed to show that while cardiac and gastrointestinal diseases may co-exist, a gastrointestinal disorder may present with features of cardiac disease and vice versa. Treatment of the underlying condition is appropriate, often resolving the symptoms completely. It is important for gastroenterologists and cardiologists to be aware of such interactions and to co-operate closely in the management of these patients (figure 2).
Acknowledgement
We are grateful to Dr Bobby Prasad, Consultant Gastroenterologist, for his help with the preparation of this paper.
Conflict of interest
None declared.
Key messages
- When evaluating typical and atypical chest pain, care should be taken to evaluate both cardiac and gastrointestinal symptoms
- A trial of both cardiac and gastro-enterological medication should be considered
- Further evaluation of both the cardiac and gastrointestinal symptoms should be considered even when a diagnosis of angina/gastro-oesophageal reflux disease is made
A comment from primary care
While we were watching rugby recently a gynaecologist friend of mine was mourning the demise of the hospital general physician. This paper highlights the interconnectedness of medicine. We already know that chronic cough may be due to gastro-oesophageal reflux disease (GORD). Most general practitioners will also have come across patients with proven coronary disease whose exertional angina disappears with a proton-pump inhibitor (PPI). This paper brings to our attention various associations between gastrointestinal and cardiac disease. It also outlines the evidence, as it stands, for possible causal links.
The most interesting parts for a general practitioner probably relate to the link between GORD and angina, and the possible association between irritable bowel syndrome (IBS) and autonomic-mediated palpitations.
The idea that GORD may actually cause ischaemic cardiac pain through reflexly induced microvascular spasm, while speculative, is very interesting. Some decades ago I worked for a bronzed Italian professor of cardiology who was fascinated by microvascular spasm. His enthusiasm (and possibly his urbane dress sense) persuaded me that my plumber’s model of angina had limitations.
In primary care, GORD is usually ‘diagnosed’ on the basis of a therapeutic response to a PPI. There is much to be said for this type of therapeutic pragmatism. But, like elegant biological models, it has its limitations.
This paper points out quite a lot of interesting associations between gastrointestinal and cardiac disease. It postulates several interesting biological models to explain the associations. However, I am aware that my own enthusiasm for plausible biological models needs to be tempered by that half-remembered image of the middle-aged man with arching ST segments clutching his Gaviscon.
Conflict of interest
None declared.
Kevin Barraclough
General Practitioner
Hoyland House Surgery, Hoyland House, Gyde Road, Painswick, Stroud, GL6 6RD (k.barraclough@btinternet.com)
References
- Kakuchi H, Sato N, Kawamura Y. Swallow syncope associated with complete atrioventricular block and vasovagal syncope. Heart 2000;83:702–04.
- Elam MP, Laird JR, Johnson S, Stratton JR. Swallow syncope associated with complete atrioventricular block: a case report and review of the literature. Military Medicine1989;154:465–6.
- Sapru RP, Griffiths PH, Guz A, Eisele J. Syncope on swallowing. Br Heart J1971;33:617–22.
- Bajaj S, Ragaza E, Silva H. Deglutition tachycardia. Gastroenterol 1972;62:632–5.
- Wilmshurst PT. Tachyarrhythmias triggered by swallowing and belching. Heart1999;81:313–15.
- Palmer ED. The abnormal upper gastrointestinal vagovagal reflexes that affect the heart. Am J Gastroenterol 1976;66:513–22.
- Terasaka R, Takemoto M, Haraoka S. Swallowing-induced paroxysmal supraventricular tachycardia. Jpn Heart J 1987;28:555–60.
- Garcia-Pulido J, Patel PH, Hunter WC, Douglas JE, Thomas E. Esophageal contribution to chest pain in patients with coronary artery disease. Chest 1990;98:806–10.
- Serebro HA. The prognostic significance of the viscerocardiac reflex phenomenon. S Afr Med J 1976;50:769–72.
- Smith KS, Papp C. Episodic, postural, and linked angina. BMJ 1962;2:1425–30.
- Morris JC, Shelburn PF et al. Coronary disease and hiatus hernia. JAMA 1963;183:788–90.
- Davies HA, Page Z, Rush EM, Brown AL, Lewis MJ, Petch MC. Oesophageal stimulation lowers exertional angina threshold. Lancet 1985;1:1011–14.
- Manfrini O. Coronary spasm reflects inputs from adjacent esophageal system. Am J Physiol Heart Circ Physiol 2006;290:H2085–H2091.
- Ballinger A, Farthing MJ. Ulcerative colitis complicated by Wenckebach atrioventricular block. Gut 1992;33:1427–9.
- Manomohan V, Subbuswamy SG, Willoughby CP. Crohn’s disease and pericarditis. Postgrad Med J 1984;60:682–4.
- Fear JD, Hutton WN. Ulcerative colitis complicated by complete heart block. BMJ1985;291:143.
- Wik B, Hillestad L. Deglutition syncope. BMJ 1975;3:747.
- Deguchi K, Mathias CJ. Continuous haemodynamic monitoring in an unusual case of swallow induced syncope. J Neurol Neurosurg Psych 1999;67:220–2.
- Keidar S, Grenadier E, Fleischman P, Palant A. Swallowing induced atrial tachycardia and fibrillation in a patient with a Wolf-Parkinson-White syndrome. Am J Med Sci1984;288(1):32–4.
- Morady F, Krol RB, Nostrant TT, De Buitleir M, Cline W. Supraventricular tachycardia induced by swallowing: a case report and review of the literature. Pacing Clin Electrophysiol 1987;10(1 Pt 1):133–8.
- Suarez LD, Chiozza MA, Foye R, Mosso H, Perosio AM. Swallowing-dependent atrial tachyarrhythmias. Their mechanism. J Electrocardiol 1980;13:301–05.
- Shirayama T, Inoue D, Omori I, Ueda M, Katsume H, Nakagawa M. Swallowing-induced tachycardia; three modalities of autonomic nervous effects. Jpn J Med 1989;28:647–50.
- Burton JR, Sachs HJ, Keon WJ, FitzGibbon GM. Intrapleural positioning of esophagus for treatment of swallowing-induced arrhythmia. Chest 1981;79:367–8.
- Schofield PM, Bennett DH, Whorwell PJ et al. Exertional gastro-oesophageal reflux: a mechanism for symptoms in patients with angina pectoris and normal coronary angiograms. BMJ 1987;294:1459–61.
- Harford WV. Southwestern Internal Medicine Conference: the syndrome of angina pectoris: role of visceral pain perception. Am J Med Sci 1994;307:305–15.
- Kramer P, Hollander W. Comparison of experimental esophageal pain with clinical pain of angina pectoris and esophageal disease. Gastroenterology 1955;29:719–43.
- Mellow MH, Simpson AG, Watt L, Schoolmeester L, Haye OL. Esophageal acid perfusion in coronary artery disease. Induction of myocardial ischemia. Gastroenterology 1983;85:306–12.
- Von Bergmann G. Das ”epinephrale syndrom” seine beziehung zur Angina Pectoris und zum kardiospasmus. Deutsch Med Wschr 1932;58:605–09.
- Chauhan A, Petch MC, Schofield PM. Effect of oesophageal acid instillation on coronary blood flow. Lancet 1993;341:1309–10.
- Chauhan A, Mullins PA, Taylor G, Petch MC, Schofield PM. Cardioesophageal reflex: a mechanism for “linked angina” in patients with angiographically proven coronary artery disease. J Am Coll Cardiol 1996;27:1621–8.
- Meredith IT, Yeung AC, Weidinger FF. Role of impaired endothelium-dependent vasodilation in ischaemic manifestations of coronary disease. Circulation 1993:87(Suppl V):56–66.
- Hick DG, Morrison JF, Casey JF, al-Ashhab W, Williams GJ, Davies GA. Oesophageal motility, luminal pH, and electrocardiographic-ST segment analysis during spontaneous episodes of angina like chest pain. Gut 1992;33:79–86.
- Lam HG, Dekker W, Kan G, van Berg Henegouwen GP, Smout AJ. Esophageal dysfunction as a cause of angina pectoris (“linked angina”): does it exist? Am J Med1994;96:359–64.
- Valori RM. Nutcracker, neurosis, or sampling bias? Gut 1990;31:736–7.
- Smart HL, Atkinson M. Abnormal vagal function in irritable bowel syndrome. Lancet1987;2:475–8.
- Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand’s disease the link? Lancet 1992;340:35–7.
- Kaddoura S, Shidrawi RG, Poole-Wilson PA. Severe gastrointestinal bleeding relieved by cardiac surgery. Br J Cardiol 1995;2:23–5.
- Vincentelli A, Susen S, Le Tourneau T et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med 2003;349:343–9.

